In a chemistry lab, almost every experiment is done in glass labware, whereas in modern biology-focused labs plastic seems to be the default. Plastic labware is cheap and can often be bought pre-sterilized, but it may not be ideal for some applications.

Most organic solvents are not compatible with plastics. As a rule of thumb for the plastics used most commonly in the lab, short-chain alcohols, acetonitrile and DMSO are compatible, other organic solvents are not, and halogenated solvents are particularly bad. The details depend on the exact type of plastic used). Take a look at what acetone does to this poor styrofoam cup! (From this video by Steve Spangler). The same thing will happen if solvents come in contact with polystyrene microtiter plates or parafilm. Polypropylene (e.g. 96-deepwell plates) may look like it holds up better, but will still leach contaminants into your samples which can affect downstream analysis or bioassays. If you pipette from a bottle of solvent using a plastic serological pipette or micropipette tip, not only are you introducing plasticizers into your sample, you have ruined the bottle for all your colleagues!
Hydrophobic molecules in aqueous solutions may adsorb (“stick”) to the plastic since it preferentially interacts with the hydrophobic plastic over the water. This could cause you to under-estimate the titer of a molecule produced, or could limit the availability and effective concentration of an exogenously added molecule such as a substrate, inhibitor, or probe.
Let's take a look at what it takes to replace plastic labware with glass in practice:
Suppose you need to add an exact amount of organic solvent to a quantitative experiment, and eye-balling it using a Pasteur pipette and rubber bulb won’t do. Your micropipette is exact, but uses plastic tips. Do glass micropipette tips exist? In a way, yes. Attach a ~1.5 cm piece of Tygon tubing (e.g. the tubing you use for connecting your Bunsen burner to the gas line) snugly onto the bottom of a 1000 µL pipette; on the other side of the tubing, connect a Pasteur pipette, which now acts as your disposable micropipette tip! According to my experiments, the volumes you can pipette this way are spot-on!
Be aware of the material of the piston inside your micropipette: Volatile acids (e.g. trifluoroacetic acid) will corrode metal pistons, and volatile organic solvent are not compatible with plastic pistons. In stead of throwing out an old micropipette with a corroded piston, consider making it your dedicated solvent pipette. You’d be surprised how accurate it still is!
Glass serological pipettes are also commonplace, just use an old-fashioned manual pipette bulb (e.g. Fisher part # 13-681-102) in stead of automated pipette controllers (“pipet-aids”) since the vapors will take a toll on the latter.

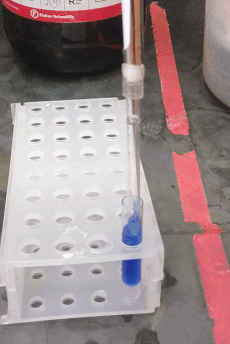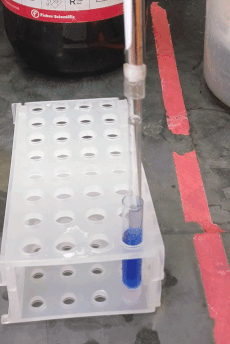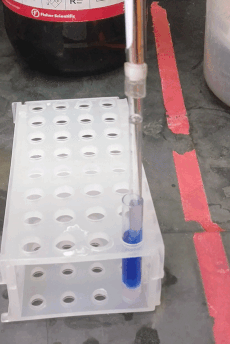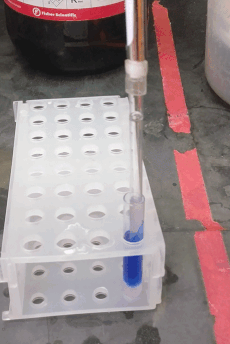
If your sample prep requires a liquid-liquid extraction step, you may want to use centrifugation to improve phase separation. You are probably familiar with the glass centrifuge tubes used in the organic chemistry lab (e.g. Fisher part # 05-569-2), which can replace 15 mL conical “Falcon” tubes, and can be centrifuged up to about 2500 × g. However, for sub-milliliter extractions (like those you might want to do for GC or LC sample prep) you may be tempted to use 1.5 mL “epi” centrifuge tubes. In stead, consider using these adorable small glass tubes (VWR part # 47729-566, picture to the right). They fit about 700 µL and can be centrifuged up to 5000 × g using a 2 mL epi tube as an adapter (see picture). They can be hard to reach inside them with regular pipette tips, but the "pasteur pipette tips" described above fit easily.

You can also perform all-glass experiments in 96-well format. There exist plastic 96-well plates that are covered in a small layer of glass (e.g., Thermo Fisher part # 60180-P300 , called “Plate +”). In my experiments, analytes that would completely adsorb onto regular polypropylene or polystyrene plates were still detectable when using these plates. Unfortunately, you cannot autoclave them (I soak them in ethanol or isopropanol overnight and let them airdry in a laminar flow hood to clean and sterilize them), and you can only centrifuge them up to 3000 × g. There also exist 96-well plates with glass inserts (e.g., WVR part # 22847613).
This certainly does not mean you should move all your experiments into glass. In many cases, glassware is more expensive (it can often be cleaned and re-used however), and some cell lines prefer to grow on specific types of plastic. Hydrofluoric acid and many other fluoride sources can dissolve glass (and your bones! If you are doing experiments with HF you ought to take serious safety precautions!). That said, next time you perform an experiment that requires a solvent extraction or are analyzing hydrophobic molecules, think about how your plastic labware may affect your experiment and consider using glassware in stead.