What is the charge state of a molecule dissolved in buffer? For the sake of simplicity let's assume you don't exceed the buffering capacity of the buffer. For molecules with a single acidic proton (or basic site), it's a simple mental exercise: when the pH of the buffer equals the pKa of the acidic proton, it's 50:50 protonated:deprotonated, at a lower pH it's protonated and at a higher pH it's deprotonated. But if you have multiple exchangeable sites it can get less intuitive. The software MarvinSketch (https://chemaxon.com/products/marvin, free for non-commercial use) has a built-in tool that can do these kinds of calculations for you. Go to Calculations -> Protonation -> pKa, pick “macro” for “mode” and make sure “Show distribution chart” is checked.
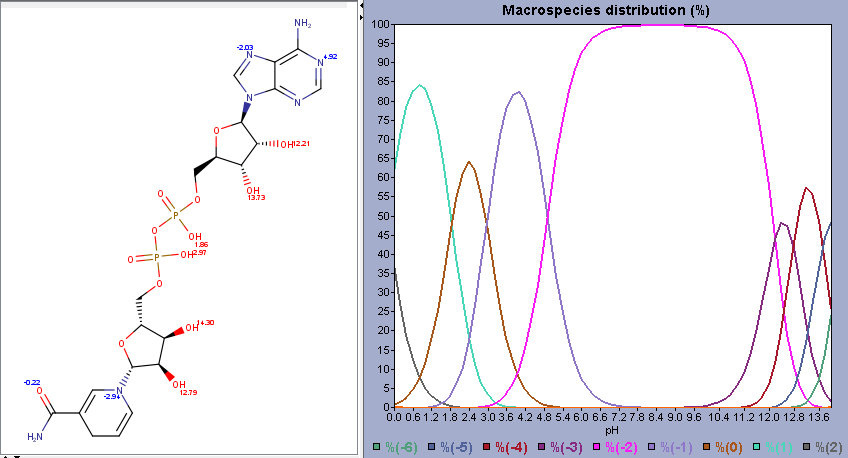
This will generate the following interactive plot, with predicted pKa values plotted on the left and the distribution plot on the right. As always with predictions: take it with a grain of salt, especially when dealing with “unusual” protons that the model may not be thoroughly trained on.
MarvinSketch can do a lot of other useful calculations. Try playing around with it!